Prediction of Patient Deterioration on the General Care Floor: Making the Connection with ICU Admissions & Resource Utilization – A Special Series on Perioperative Patient Safety
Introduction
A late-night code blue alarm ringing through a hospital’s hallways is not new to our ears. We as anesthesiologists and intensivists have been ‘first responders’ to these adverse events and also ‘first receivers’ of these patients in the ICU. This ‘4am’ patient deterioration phenomenon may be a simple lack of appropriate surveillance systems or maybe a more complex interplay of underlying patient physiology and concurrent disease insults.1 In this issue of the SOCCA Interchange, we continue our series on perioperative cardiorespiratory events outside the ICU with a look at the intricate relationship between ICU admission and resource utilization. Dr(s). Wongtangman and Eikermann answer two seemingly simple but actually challenging questions. First – which patients are likely to need ICU admission postoperatively, and how do we identify these individuals accurately ahead of time before they are sent to the floor and come back as a code 12 hours later. Second – what should we do to better allocate our limited resources to improve postoperative surveillance and upstream treatment. This discussion offers much-needed insight into issues that affect preventable ICU admissions and establishes a strong connection between our work inside the ICU, in the operating room, in the PACU, and beyond those walls. More than half of all adverse events in hospitalized patients occur outside the ICU. About 40% of patients sustaining index cardiorespiratory events on hospital floors die before they leave the hospital.2 Needless to say, the hospital ward, though perceived as a low-acuity environment, is actually a common venue for critical events during a period in which patients are especially prone to developing clinical deterioration and life-threatening complications.3,4 Dr(s). Wongtangman and Eikermann take us as close to a ‘crystal ball’ as possible and help us understand the answer to the one PACU question that is always on our mind: “Does this patient need to go to the ICU now?”
- Ashish K. Khanna, MD, FCCP, FCCM
Patient deterioration after surgery can be defined as an objective derangement in patients’ clinical status (e.g. vital signs, neurological examination) combined with findings that may be more subjective (e.g. agitation, feeling unwell).5,6 The reported incidence of postoperative deterioration within three days after surgery is nearly 30%, and the contributing mechanisms are likely multifactorial.7,8 Undetected acute deterioration can cause serious adverse events. A recent European multi-center study demonstrated that the in-hospital mortality rate of patients undergoing non-cardiac surgery was higher than anticipated at about 4%.9 Interestingly, 73% of patients who died in the hospital were not admitted to an intensive care unit (ICU) at any stage after surgery.9
This raises two questions. First, how do we identify the patients who may be treated in high resource settings, such as an ICU, without a clearly defined need, since these resources are limited, expensive, and can only be justified if they lead to better outcomes. Second, how should we triage the additional resources required for postoperative surveillance and treatment?
Which patients should directly go to the ICU after surgery?
Without an absolute indication for ICU admission, such as mechanical ventilation, it remains unclear which patients may benefit from postoperative critical care. Several recently published studies focus on the issue of inadequate ICU admission criteria: that we treat some patients in the ICU without medical need and do not admit others who need ICU care based on objective criteria. In an observational study, Wunsch et al. examined administrative data from 7,878 Medicare patients who underwent major surgery at 162 hospitals. They found that higher rates of perioperative intensive care did not translate to reduced mortality, cost, or length of stay.10 In contrast, routine admission to the ICU after a specific type of surgery alone caused longer hospital stays and higher costs. In an observational study of 3,530 matched patients undergoing non-cardiac surgery in a health care network in New England, our group examined whether postoperative admission to an ICU versus surgical ward affected hospital length of stay and cost. Among surgical patients with a low likelihood of postoperative ICU admission (adjusted by patient, surgical, and intraoperative factors), initial triage to an intensive care unit was associated with increased postoperative hospital length of stay and costs. By contrast, for patients with a high likelihood of postoperative ICU admission, triage from the operating room to the ICU was associated with decreased postoperative hospital length of stay and costs.11 We concluded that straightforward, healthy patients who go to the ICU are problematic. On the contrary, the treatment of complex patients who do not get postoperatively admitted to ICU is more expensive. This supports European data indicating that patients secondarily admitted to the ICU after initial care on a ward had a higher risk of death when compared with patients directly admitted to the ICU after surgery.12
Based on these data, scoring systems should be used to aid decision making for postoperative bed allocation in order to place patients at their most appropriate level of care. For example, The Score for the Prediction of Postoperative Respiratory Complications (SPORC-2) is a simple prediction model for postoperative tracheal re-intubation. The score is comprised of five pre-operative variables and seven intra-operative variables, which could be used by clinicians to identify at-risk patients.13 In addition, other scoring systems created for postoperative prediction of morbidity and mortality, such as the Portsmouth Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (P-POSSUM)14 or the surgical agar score (SAS),15 can be applied to aid patient allocation. Moreover, clinician scientists can use their own data to create a score for prediction of ICU admission. We have created such a model at the Massachusetts General Hospital containing 23 variables, which included patient demographics (age, sex, body mass index, Charlson Comorbidity Index, ASA physical status), surgical characteristics (principal surgical procedure, emergency status, duration of surgery, high-risk surgery, procedural complexity), intraoperative physiologic measures (estimated blood loss, duration of hypotension, median heart rate, median positive end- expiratory pressure, median plateau pressure, and median arterial oxygen saturation/fraction of inspired oxygen ratio), and intraoperative drug and fluid therapy (vasopressor and neuromuscular blocking agent doses, colloid and crystalloid).11
In summary, to use objective criteria that include comorbidities, procedural risk factors, and vital signs for triage decreases costs and improves outcomes in patients after major surgery.
Which patients need to be screened and possibly treated postoperatively on the surgical ward
While ‘clinically stable’ patients are typically admitted to a general care ward after surgery, nearly half of all adverse events occur on the ward.16 Progressive respiratory and circulatory compromise are the most common causes of deterioration – these indicators often become visible several hours before culmination in a clinically meaningful adverse event.17,18 ‘Failure to rescue,’ that is death following a complication,19 may occur when early indicators of postoperative deterioration go unrecognized. Failure to rescue can be possibly prevented using quality assurance interventions. In an observational study of 269,911 patients, the authors showed that hospitals with low and high mortality had a similar complication rates. However, the high-mortality hospital group carried higher failure to rescue rates.20
The American Heart Association highlights a system of appropriate surveillance to prevent in-hospital cardiac arrest as a ‘first link’ in the chain of survival.21 Rapid response (i.e., medical emergency) teams provide emergency assistance to deteriorating patients and form a cornerstone of traditional patient safety systems on general care wards.22 The classic model includes an afferent limb – that detects the event and triggers a systematic response – and the efferent limb – that provides resources to stabilize and triage the patient to a location where services meet the patient’s needs.23
Several early warning tools have been proposed to trigger rapid response systems. These tools include the Modified Early Warning Score,24 the National Early Warning Score,25 and other clinical criteria for activating a medical emergency team response.26 However, the optimal approach and alerting thresholds remain elusive. Monitoring vital signs is an essential step in detecting deteriorations in all these tools. To activate the rescue system more promptly, patient monitoring needs to be systematically improved and more intensive. To that end, various continuous monitoring systems have been introduced into the afferent limb. Isono et al. demonstrated one such approach.27 Using four load cells placed under the bed legs, contactless respiratory measurement was achieved by capturing associated shifts in the center of gravity in human subjects in different positions such as supine; left lateral; right lateral; and 30, 45, and 60° sitting postures. Installation of such a bed sensor vital sign monitoring system could help identify patients’ postoperative deterioration when integrated in a telemedicine approach (Figure 1). Its applicability to a large population at risk of postoperative deterioration is a promising method to improve surveillance and prevent unexpected death from acute respiratory dysfunction.28
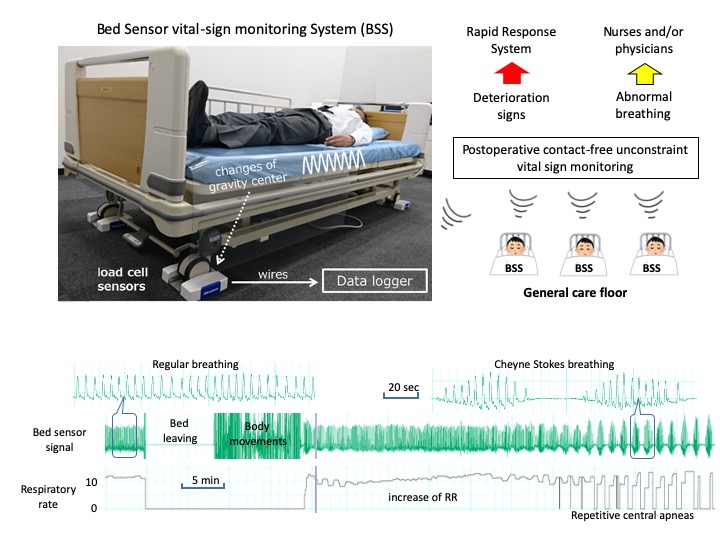
Figure 1
In conclusion, an appropriate monitoring system should be applied to facilitate prompt detection of early warning signs, so that proper management of postoperative deterioration can be triggered. Such an approach may reduce the need for higher acuity care, reducing hospital lengths of stay and admission costs while improving survival.
Take home message:
- It is not “safe” to routinely admit patients to the ICU without clear indication.
- It is not “safe” to admit patients to a general care ward, who would otherwise get admitted to an ICU based on established institutional processes, due to lack of bed availability.
- Use objective instruments (i.e., scores) to identify the proper level of care in patients at risk of deterioration admitted to the surgical floors.
- Leverage digital health opportunities for surveillance.
- Create and implement robust inter-professional processes to identify and treat patients who demonstrate signs and symptoms of unexpected postoperative deterioration.
References
- Khanna AK, Hoppe P, Saugel B. Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care. 2019;23(1):194.
- Morrison LJ, Neumar RW, Zimmerman JL, et al. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127(14):1538-1563.
- Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999-2005. Anesthesiology. 2009;110(4):759-765.
- Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059-1065.
- Guinane JL, Bucknall TK, Currey J, Jones DA. Missed medical emergency team activations: tracking decisions and outcomes in practice. Crit care Resusc. 2013;15(4):266-272.
- Vincent JL, Einav S, Pearse R, et al. Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol. 2018;35(5):325-333.
- Trinkle RM, Flabouris A. Medical reviews before cardiac arrest, medical emergency call or unanticipated intensive care unit admission: Their nature and impact on patient outcome. Crit Care Resusc. 2012;13(3):175-180.
- Mohammed Iddrisu S, Considine J, Hutchinson A. Frequency, nature and timing of clinical deterioration in the early postoperative period. J Clin Nurs. 2018;27(19-20):3544-3553.
- Pearse RM, Moreno RP, Bauer P, et al. Mortality after Surgery in Europe: A 7 Day Cohort Study. Lancet. 2012; 380(9847):1059-65.
- Wunsch H, Gershengorn HB, Cooke CR, et al. Use of Intensive Care Services for Medicare Beneficiaries Undergoing Major Surgical Procedures. Anesthesiology. 2016;124(4):899-907.
- Thevathasan T, Copeland CC, Long DR, et al. The Impact of Postoperative Intensive Care Unit Admission on Postoperative Hospital Length of Stay and Costs: A Prespecified Propensity-Matched Cohort Study. Anesth Analg. 2019;129(3):753-761.
- Gillies MA, Harrison EM, Pearse RM, et al. Intensive care utilization and outcomes after high-risk surgery in Scotland: A population-based cohort study. Br J Anaesth. 2017;118(1)123-131.
- Lukannek C, Shaefi S, Platzbecker K, et al. The development and validation of the Score for the Prediction of Postoperative Respiratory Complications (SPORC-2) to predict the requirement for early postoperative tracheal re-intubation: a hospital registry study. Anaesthesia. 2019;74(9):1165-1174.
- Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg. 1998;85(9):1217-1220.
- Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar Score for Surgery. J Am Coll Surg. 2007; 204(2):201-8.
- De Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: A systematic review. Qual Saf Health Care. 2008;17(3):216-23.
- Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-7.
- Girotra S, Nallamothu BK, Spertus JA, et al. Trends in Survival after In-Hospital Cardiac Arrest. N Engl J Med. 2012;367(20):1912-20.
- Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and Patient Characteristics Associated with Death After Surgery. Med Care. 1992;30(7):615-29.
- Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250(6):1029-34.
- Kronick SL, Kurz MC, Lin S, et al. Part 4: Systems of care and continuous quality improvement: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S397-413.
- Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-46.
- DeVita MA, Bellomo R, Hillman K, et al. Findings of the First Consensus Conference on Medical Emergency Teams. Crit Care Med. 2006;34(9):2463-78.
- Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a Modified Early Warning Score in Medical Admissions. QJM. 2001:94(10)521-6.
- Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-70.
- DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Heal Care. 2004;13(4):251-4.
- Isono S, Nozaki-Taguchi N, Hasegawa M, et al. Contact-free unconstraint respiratory measurements with load cells under the bed in awake healthy volunteers: breath-by-breath comparison with pneumotachography. J Appl Physiol. 2019;126(5):1432-1441.
- Schaefer MS, Eikermann M. Contact-free respiratory monitoring using bed wheel sensors: a valid respiratory monitoring technique with significant potential impact on public health. J Appl Physiol. 2019; 126(5): 1430-1.






































