
Invasive Fungal Infections and Immune-Mediated Hemolysis in Liver Transplantation: Two Complex Cases
by Wing Fei Wong, MD and Avneep Aggarwal, MD
Liver transplantation is a life-saving procedure but comes with significant post-operative risks, including opportunistic infections and immune-mediated complications. In this report, we present two unique cases that highlight the challenges faced in liver transplant recipients. The first case involves a rare and life-threatening gastrointestinal mucormycosis infection, emphasizing the importance of early detection and aggressive management in fungal infections. The second case explores Passenger Lymphocyte Syndrome, an often-overlooked immune complication leading to hemolysis due to minor ABO mismatch. These cases underscore the need for heightened awareness and timely intervention in transplant medicine to improve patient outcomes.
Case 1 - Invasive Fungal Infections in Liver Transplant Patients: Lessons from a Rare Case of Gastrointestinal Mucormycosis
INTRODUCTION
Gastrointestinal mucormycosis is a rare and potentially fatal manifestation of invasive fungal infection caused by organisms of the order Mucorales. It accounts for only about 7% of all mucormycosis cases yet carries mortality as high as 85%1. The stomach is the most frequently affected site, followed by the colon and small intestine. This condition primarily affects immunocompromised individuals, including those with uncontrolled diabetes, hematologic malignancies, or organ transplants. Due to its nonspecific symptoms and rapid progression, diagnosis is often delayed, with only 25% of cases identified antemortem2 . We present a case of intraabdominal mucormycosis due to a perforated gastric ulcer in a liver transplant patient.
DESCRIPTION
A 59-year-old female with a history of GERD and alcohol use presented with abdominal pain and was diagnosed with perforated gastric ulcer. During surgical repair, the portal triad was inadvertently transected, leading to fulminant liver failure. She was admitted to our ICU and supportive care was continued. She required continuous hemodialysis for acute renal failure and large volume plasmapheresis as a bridge to liver transplantation. She was urgently listed for liver transplantation, which was successfully performed.
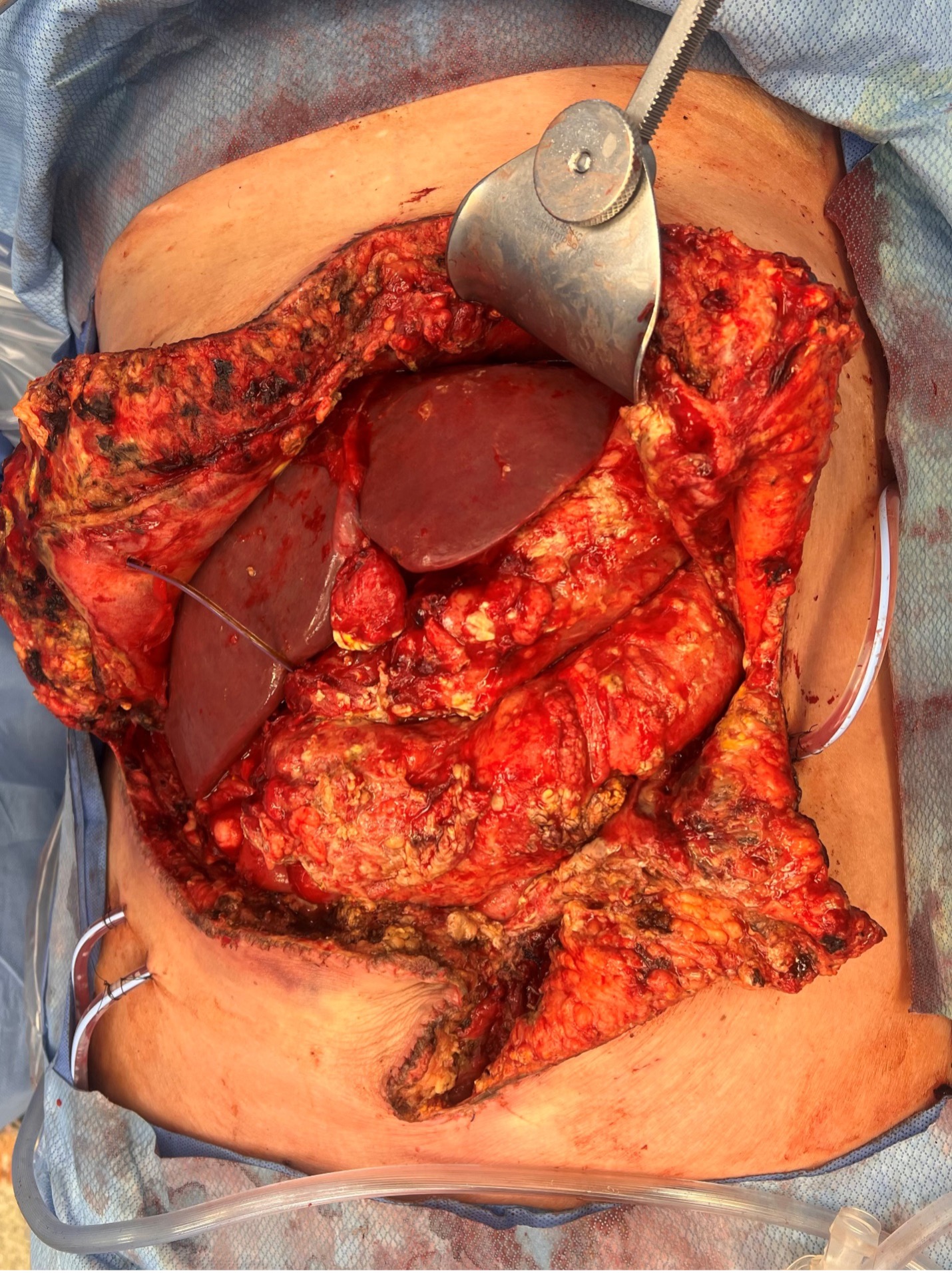
Following surgery, the patient’s post-operative course was complicated by the development of an invasive fungal infection caused by Mucorales Circinelloides. This infection was identified by intraoperative ascitic fluid cultures. Gastric ulcer biopsy at the time of transplantation demonstrated chronic gastritis. The source of the infection remained unknown. However, Infectious Disease (ID) hypothesized that Mucorales Circinelloides was present as an environmental contaminant in her GI tract, with her gastric perforation and subsequent immunocompromise from her liver injury resulting in dissemination. On post- transplant day 6, she underwent exploratory laparotomy, which found multiple areas of fungal deposits over the stomach and transverse colon, with a frozen biopsy positive for hyphae (Figure 1). Intra- abdominal drains were placed for amphotericin irrigation. Continuous intraabdominal amphotericin irrigation and serial surgical washouts every third day were employed to control the infection. She was treated with intravenous liposomal amphotericin B and posaconazole per ID recommendations. Eventually, the patient demonstrated clinical improvement, with the resolution of fungal growth and negative ascitic cultures. Early recognition and aggressive treatment were crucial for a successful outcome for our patient.
CONCLUSION Invasive fungal infections pose a significant threat to liver transplant recipients, with Candida spp., Cryptococcus, and Aspergillus species being the most prevalent pathogens. While the incidence of these infections is relatively low at 1-4%, their associated mortality rates are alarmingly high, ranging from 54.5% to 77%3. Gastrointestinal mucormycosis is a rare but lethal form of infection, with reported mortality rates reaching 85%. Its nonspecific clinical presentation complicates timely diagnosis, often resulting in identification only during surgery or postmortem. Treatment strategies primarily involve the use of liposomal amphotericin B, but its toxicity necessitates careful monitoring due to potential renal impairment and adverse drug interactions.
In our case, intraoperative culture led to the early identification of mucormycosis, enabling prompt initiation of treatment with both liposomal amphotericin B and posaconazole, along with surgical intervention and continuous irrigation. This proactive approach underscores the importance of early recognition and aggressive management in improving outcomes for patients with this formidable infection. Our experience not only adds to the limited literature on intraabdominal mucormycosis but also highlights the critical need for heightened awareness and vigilance in the management of opportunistic infections in liver transplant patients.
REFERENCES:
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005 Sep 1;41(5):634-53.
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012 Feb;54 Suppl 1:S23-34.
- Khalid M, Neupane R, Anjum H, Surani S. Fungal infections following liver transplantation. World J Hepatol. 2021 Nov 27;13(11):1653-1662.
Case 2 - Passenger Lymphocyte Syndrome: A Critical Complication Post-Liver-Transplant
INTRODUCTION
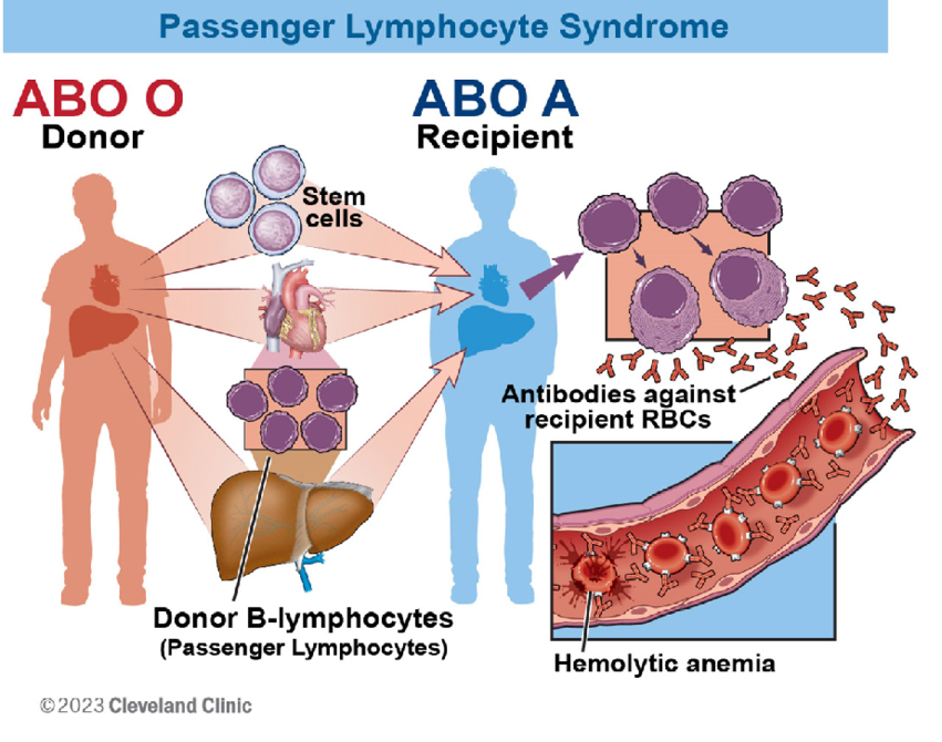
Passenger Lymphocyte Syndrome (PLS) is a limited form of graft-versus-host disease in which donor B lymphocytes are passively transferred to the recipient within the donor allograft and forms antibodies that result in complement- mediated red blood cell destruction1. It is most often seen in solid organ transplantation with minor ABO mismatch, such as an O donor with an A recipient, leading to production of anti-A antibodies. Here, we present a unique case of PLS post-emergent liver transplant in our patient.
DESCRIPTION
A 59-year-old AB+ female with past medical history significant for gastric reflux and alcohol use presented to an outside hospital with a perforated gastric ulcer. She underwent exploratory laparotomy, with accidental transection of her portal triad, leading to fulminant liver failure. She was transferred to our institution for expedited liver transplantation evaluation. Five days following her initial surgical insult, she underwent emergent liver transplantation with an A+ donor liver.
Her post-operative course was complicated by intraabdominal infection, leading to multiple washouts, necessitating repeated large volume transfusions. Post-transplant day 7, her hemoglobin dropped from 10.4 mg/dL to 6.5 mg/dL. Diagnosing the cause of anemia in our patient was quite challenging due to multiple potential underlying factors. She had not been initiated on new antibiotic therapy or started on other medications. The physical exam did not demonstrate signs of bleeding, including clean dressings, soft abdomen, and mild drain output. Though she went for surgical washout two days prior, it was uneventful, with minimal bleeding. She received 3u packed red blood cells, with rise to 9.6 mg/dL, which fell shortly to 7.7 mg/ dL. It was noted that bilirubin rose from 3.0 mg/dL to 6.8 mg/dL, suggesting a hemolytic process. Overnight, her type and screen returned as “Indeterminant ABO,” which prompted us to pursue additional testing as recommended by Transfusion Medicine. Subsequent tests demonstrated a positive direct antiglobulin test (DAGT Polyspecific AHG and anti-IgG) and eluted anti-A antibody. However, as the patient had previously been AB+, the presence of an anti-A antibody was cause for concern.
Upon review of the donor’s medical record, it was found that the donor was an A2 blood type while the recipient was A1B, leading to an atypical minor ABO mismatch. She was subsequently transfused with O-negative blood, with an appropriate transfusion response. Following a discussion with Transfusion Medicine, she was determined to have Passenger Lymphocyte Syndrome. Management included administering blood transfusions using antigen-negative or donor-type blood. This helped mitigate the effects of hemolysis by providing compatible red blood cells while minimizing the risk of further immune reactions, stabilizing the patient’s condition.
CONCLUSION
Passenger Lymphocyte Syndrome (PLS) is a rare yet significant complication that intensivists need to be aware of in patients who have undergone solid organ transplantation. It involves immune- mediated hemolysis caused by donor B-lymphocytes producing antibodies against the recipient’s red blood cells (Figure 2). Risk factors include minor ABO mismatches, specific organ transplants (liver, kidney, heart-lung), immunosuppression choice, and post-transplant infections or transfusions. PLS typically manifests 1-3 weeks post- transplant with sudden hemolysis, anemia, jaundice, and a positive direct antiglobulin test. Severity ranges from mild to life- threatening, potentially causing graft failure or disseminated intravascular coagulation.
While rare, PLS should be considered in cases of acute- on-chronic anemia post-transplant, especially with minor ABO incompatibility. Liver transplant recipients often have anemia due to chronic liver disease, surgical losses, and perioperative fluid shifts. Our case represents a unique case of PLS due to A1 and A2 subtypes of blood group A. About 80% of individuals with A antigen are A1, while 20% are A2 or rarer subtypes. Of A2 individuals, only 0.4 % have anti-A12 . Treatment is mainly supportive, including compatible blood transfusions, increased immunosuppression, corticosteroids, and possible rituximab or plasmapheresis in severe cases. As an intensivist, maintaining a high index of suspicion for PLS in post-transplant patients presenting with unexplained anemia or hemolysis is crucial for timely diagnosis and management.
REFERENCES:
- Brunetta DM, Albuquerque LM, Batista AHM, Santos LHO, Schreen D, Lima CA, Mesquita DFG, Carlos LMB, Garcia JHP. Passenger lymphocyte syndrome in liver transplantation. Rev Bras Hematol Hemoter. 2017 Oct-Dec;39(4):364-367.
- Giriyan SS, Agrawal A, Bajpai R, Nirala NK. A1 and A2 Sub-Types of Blood Group ‘A’: A Reflection of their Prevalence in North Karnataka Region. J Clin Diagn Res. 2017 May;11(5):EC40-EC42. doi: 10.7860/ JCDR/2017/26772.9893. Epub 2017 May 1. PMID: 28658771; PMCID: PMC5483673.
- Barouqa, Mohammad & Jum’ah, Husam & Janbek, Yousef & Reyes, Morayma. (2023). Passenger lymphocyte syndrome from Transfusion Medicine’s standpoint: Short Communication. JAP Academy Journal. 1. 10.58877/japaj.v1i2.66.