Artificial Intelligence in Neuro-Critical Care Settings
Introduction:
Neuro-critical care constitutes a demanding, high-alert setting that requires expeditious decision-making by critical care teams in response to patient health status. The resource-intensive nature of neuro-critical care settings results in a substantial financial burden. As of 2016, the Centers for Disease Control and Prevention (CDC) reported an annual national healthcare spending cost of $40.6 billion for non-fatal Traumatic Brain Injuries (TBI), with Medicare-insured patients contributing more than 50% of this figure.1 Integrating artificial intelligence (AI) into medical applications has witnessed exponential growth, assisting with clinical decision-making. This article highlights the basis of AI learning and its integration into neuro-critical care settings and discusses potential AI applications within the neuro-critical care environment.
Artificial intelligence:
Artificial intelligence (AI) is the simulation of human intelligence processes by machines, especially computer systems. In the medical field, the use of AI has become more prevalent, and it has evolved to revolutionize diagnosis, treatment plan, and patient care through the analysis of vast amounts of data to assist healthcare professionals in making more accurate and efficient decisions, ultimately leading to improved patient care and outcomes.
AI learning is the process by which the machines analyze data, identify patterns, and adapt their algorithms to improve performance without explicit programming. AI learning is broadly categorized into two schools of thought: supervised and unsupervised learning.
Supervised learning relies on manually inserted or “labeled” information and specific criteria to create an algorithm that produces the output from the available data. The output in supervised learning is pre-determined, and the algorithms are responsible for rapidly categorizing and streamlining the data to one of the desired outputs. In a medical context, a supervised learning algorithm is analogous to the patient interviewing and medical decision-making process. The “inputs” are the symptoms, pertinent history, or exam findings, and the outputs can range from a broad differential to precise diagnoses.2 This medical decision-making process mirrors the function of a random forest and decision tree analysis algorithm (further explained in Figure 1).3 For example: a woman wants to run outside, but she does not want to run if the ground is wet from a recent storm or if the temperature is freezing cold. The algorithm here would process each criterion as individual decisions. The last storm was 24 hours ago, and the ground appears dry, so the algorithm could set a threshold of “12 hours since previous storm” to approximate if the ground will be wet. Thus, the algorithm gives “true” as its output and proceeds to the next decision. If the threshold was instead set at “48 hours since the previous storm”, the output would be “False,” and the algorithm would default to the woman ‘not running.’ Supervised learning estimates only what it is trained to do based on clearly defined thresholds and outputs.
In contrast, unsupervised learning has unique outputs, thus allowing the algorithm to generate responses not explicitly programmed during its training. Artificial neural networks, one of the most common subtypes of unsupervised algorithms, are designed with multiple layers of interconnecting data nodes that interact, like the numerous layers of interconnecting neurons in the nervous system. The activation of nodes in hidden layers is determined by threshold values, which can fluctuate depending on how frequently a specific connection is activated or bypassed.4 The addition of variables, such as environmental conditions, creates additional nodes that can broaden the number of potential outputs and potential connections within the algorithm. Figure 1 depicts the variety of supervised and unsupervised algorithms with brief explanations of each function.
Integration of AI in Neurocritical Care:
Example 1: AI and ICP management
The integration of AI applications within neurocritical care can provide substantial benefits. The time-sensitive nature of the neurocritical care environment necessitates continuous surveillance using a diverse number of data-gathering equipment. Intracranial Pressure (ICP) is one such variable. ICP requires paramount supervision due to its robust predictive value for mortality.6 Elevated ICP can rapidly lead to cerebral hypoperfusion and herniation if not treated or if it doesn’t respond quickly to medical management. ICP is an invaluable variable for Multi-Modality Monitoring (MMM).2 MMM entails recruiting and combining data for similar variables from multiple measurement devices to improve monitoring precision and aid in promptly detecting fluctuations in clinical conditions to perform medical decision-making.5
Traditionally, the gold standard for ICP measurement is intraparenchymal strain gauges or fiber-optic monitors. Other modalities include ultrasound measurements of optic nerve sheath diameter (ONSD) or ventricular diameter, but neither has demonstrated accuracy for clinical decision-making.6 The true potential of AI algorithms in ICP monitoring lies in their capacity to predict impending changes. Notably, Zweifel et al. delved into the application of the Cerebrovascular Reactivity Index (CRI) to assess the brain’s autoregulatory function in response to variations in cerebral blood pressure.7 In normal circumstances, cerebral arterioles vasoconstrict in reaction to elevated blood pressure, thus reducing excessive cerebral perfusion and increasing ICP. Consequently, decreased perfusion leads to reduced ICP, establishing an inverse relationship between ICP and arterial pressure. In cases of impaired cerebral reactivity, as observed in traumatic brain injuries (TBI), this relationship becomes direct, meaning any rise in blood pressure may result in a subsequent increase in ICP.7 Figure 2 portrays pulse waveforms illustrating normal and impaired cerebral reactivity, with CRI calculated as the correlation coefficient at 8-10 second intervals. In this study and in the clinical setting, measuring ICP with an intraparenchymal gauge allows frequent measurements and easy trending of data points. Intraparenchymal measurements also provide global information instead of local cerebral ICP data, which is a limitation of arterial Doppler analysis.6 However, the scope of AI integration in ICP monitoring extends beyond trend analysis. An unsupervised algorithm utilizing CRI can track ICP variability, alerting when there is a high probability of surpassing the critical threshold of 20 mmHg. An algorithm could also take an active role in the immediate treatment, such that it can be programmed to trigger immediate interventions such as elevating the head of the bed to a minimum of 30 degrees, adjusting the ventilation rate on the nearby ventilator to induce hypocarbia, or administering a measured dose of mannitol. The implementation of a swift and automated response has the potential to forestall further decompensation, affording the critical care team additional time for nuanced decision-making beyond the algorithm’s preprogrammed responses.2
Example 2: AI and EEG management
In addition to ICP, seizures contribute to the complexity and demands of neurocritical care due to numerous interventions and complications related to the event. The resource-intensive nature of seizure monitoring presents a compelling opportunity for the integration of machine learning algorithms. Presently, Electroencephalograms (EEGs) are applied to patients and continuously observed by trained technicians, who relay suspicious findings to the critical care team when epileptiform activity is seen on EEG recordings. Prompt diagnosis is essential for preventing substantial clinical deterioration, especially given the diverse etiologies that can precipitate seizures. One study found that some patients who presented with altered mental status were subsequently diagnosed with nonconvulsive status epilepticus (SE), a scenario where EEG remains the gold standard diagnostic tool capable of accurate diagnosis.8 AI holds the potential to streamline EEG interpretation for critical care teams, particularly in settings with limited resources or personnel.9 Chavakula et al. devised an algorithm for objectively quantifying interictal spikes in raw EEG samples. The algorithm, depicted in Figure 3, comprised a 5-stage design before entering a classification program that accurately discerned the lateralization of interictal spikes in single and multiple-channel EEGs across various stages of the sleep-wake cycle. The algorithm identifies a “wavelet” on an EEG strip and demonstrated a sensitivity of approximately 77.6% and specificity of approximately 87.6% when compared to spike quantification conducted by two board-certified clinical neurophysiologists.10 This study underscores the potential of machine learning in aiding the early detection of epileptic activity.
Although primarily applied for epileptiform monitoring purposes, the utility of EEG can extend into other variable monitoring such as ICP monitoring. Connoly et al. explored the correlation between EEG signal changes and ICP in two patients at the UCLA neurocritical care unit undergoing burst suppression with phenobarbital.11 ICP was measured through an extraventricular drain catheter, and depth EEG waveforms were assessed using a 6-electrode strip placed adjacent to the catheter. The study found that approximately 79.6% of bursts in EEG were followed by a transient increase in ICP. Moreover, Spearman correlation analysis revealed a significant association between the amplitude of ICP changes and the duration of bursts on EEG.11 Despite the small sample size of this study, it unveils the potential contributions of EEG in neurocritical care settings. While AI algorithms designed for ICP management can be adapted even when EEG monitoring is the primary measurement driver, current research primarily assesses directional ICP fluctuations correlated with EEG changes. A future avenue could involve investigating EEG readings at critical ICP levels, specifically those exceeding 20 mmHg, considering potential risks of cerebral ischemia, which may display generalized slowing of waveforms. This approach necessitates trend interpretation rather than the EEG providing a distinct, recognizable pattern at predefined ICP thresholds.
Concerns regarding use of AI:
AI is an exciting tool that is rapidly expanding. However, emerging technology brings emerging concerns. An international survey in 2023 amongst 650 surgeons in 71 countries considered AI to be a last resort among other resources such as guidelines, training programs, and second opinions.12 One thought is that information and education regarding AI is limited, and not enough clinicians understand how AI could help in clinical settings. In the same survey results, emergency and trauma surgeons expressed the need for sound decision aids.13 The above-mentioned examples comprise only a small percentage of all the AI projects ongoing to organize and analyze big data. The need to prepare and educate medical teams on how AI can serve their medical decision-making needs should be as paramount as programming algorithms.
Patient autonomy, confidentiality, justice, and protection are also decisive concerns with AI. AI regulation and oversight systems will be required as AI software is progressively included in routine medical care. Many large tech corporations like Google and Microsoft are developing their own AI systems for big data collection in healthcare and personalized health information, either from smart watches or other innovative devices.14 Patient competency with medical decision-making can fluctuate in neurocritical care settings, yet patient autonomy must be maintained. However, will vulnerable patients have the capacity to consent to AI’s role in their healthcare, especially if AI grows the ability to administer medicines automatically? A survey study in 2021 asked physicians and patients who they thought would be responsible if an adverse effect occurred with direct AI involvement.13 Figure 4 shows the bar graph from the study, noting that most respondents (patients and physicians) thought physicians should carry liability for Al. These questions will likely be answered as new legislation comes in and AI becomes the standard of care in the future. With that being said, the safety and autonomy of individuals must always take priority.
Conclusion:
AI is gradually bridging the gap between data collection and analysis and can serve as a key contribution to improving neurocritical care. ICP and Epilepsy-related algorithms are a few of the many disciplines that are working to elevate neurocritical care. Hospitals and clinics with limited access to resources and staffing are most likely to witness the benefits of integrating AI first. The limiting factors for further integration of AI into neurocritical care are a lack of provider education and the ethical dilemmas surrounding patient autonomy and justice. More studies are needed to look at the efficiency and reliability of AI in healthcare settings.
We have no conflicts of interest to disclose.
Correspondence concerning this article should be addressed to Vinodkumar Singh, University of Alabama at Birmingham (UAB), Birmingham, AL 35233, United States. Email: vsingh@uabmc.edu
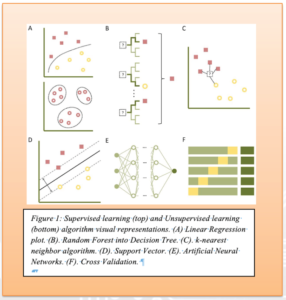
SOCCA Interchange Newsletter | Volume 35 | Issue 2 | Summer 2024 | Figure 1

SOCCA Interchange Newsletter | Volume 35 | Issue 2 | Summer 2024 | Figure 2
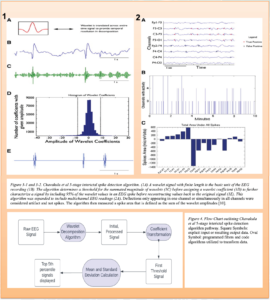
SOCCA Interchange Newsletter | Volume 35 | Issue 2 | Summer 2024 | Figure 3
References:
- Miller, G. DePadilla, L. Xu, L. Costs of non-fatal traumatic brain injury in the United States, 2016. National Center for Injury Prevention. Med Care. 2021 May 01; 59(5): 451–455. doi:10.1097/MLR.0000000000001511
- Al-Mufti F, Dodson V, Lee J, Wajswol E, Gandhi C, Scurlock C, Cole C, Lee K, Mayer SA. Artificial intelligence in neurocritical care. J Neurol Sci. 2019 Sep 15;404:1-4. doi: 10.1016/j.jns.2019.06.024. Epub 2019 Jun 29. PMID: 31302258.
- IBM. Supervised Learning. IBM.com. https://www.ibm.com/topics/supervised-learning. Accessed April 20th, 2024.
- IBM. Neural Networks. IBM.com. https://www.ibm.com/topics/neural-networks. Accessed April 20th, 2024.
- Neurocritical Care and Brain Monitoring James J. Riviello MD, Jennifer Erklauer MD. Neurologic Clinics, 2021-08-01, Volume 39, Issue 3, Pages 847-866, Copyright © 2021 Elsevier Inc.
- Munakomi S, M Das J. Intracranial Pressure Monitoring. [Updated 2023 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available
- Zweifel, C., Lavinio, A., Steiner, L. A., Radolovich, D., Smielewski, P., Timofeev, I., Hiler, M., Balestreri, M., Kirkpatrick, P. J., Pickard, J. D., Hutchinson, P., & Czosnyka, M. (2008). Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurgical Focus FOC, 25(4), E2.
- Baker AM, Yasavolian MA, Arandi NR. Nonconvulsive status epilepticus: overlooked and undertreated. Emerg Med Pract. 2019 Oct;21(10):1-24. Epub 2019 Oct 1. PMID: 31557430.
- Kamousi B, Karunakaran S, Gururangan K, Markert M, Decker B, Khankhanian P, Mainardi L, Quinn J, Woo R, Parvizi J. Monitoring the Burden of Seizures and Highly Epileptiform Patterns in Critical Care with a Novel Machine Learning Method. Neurocrit Care. 2021 Jun; 34(3):908-917. doi: 10.1007/s12028-020-01120-0. Epub 2020 Oct 6. PMID: 33025543; PMCID: PMC8021593
- Vamsidhar Chavakula, Iván Sánchez Fernández, Jurriaan M. Peters, Gautam Popli, William Bosl, Sanjay Rakhade, Alexander Rotenberg, Tobias Loddenkemper. Automated quantification of spikes. Epilepsy & Behavior, Volume 26, Issue 2, 2013, Pages 143-152, ISSN 1525-5050
- Connolly, M., Vespa, P., Pouratian, N., Gonzalez, N. R., & Hu, X. (2015). Characterization of the Relationship between Intracranial Pressure and Electroencephalographic Monitoring in Burst Suppressed Patients. Neurocritical Care, 22 (2), 212.
- Cascella M, Montomoli J, Bellini V, Vittori A, Biancuzzi H, Dal Mas F, Bignami EG. Crossing the AI Chasm in Neurocritical Care. Computers. 2023; 12(4):83. https://doi.org/10.3390/computers12040083
- Cobianchi, L., Piccolo, D., Dal Mas, F. et al. Surgeons’ perspectives on artificial intelligence to support clinical decision-making in trauma and emergency contexts: results from an international survey. World J Emerg Surg 18, 1 (2023).
- Murdoch, B. Privacy and artificial intelligence: challenges for protecting health information in a new era. BMC Med Ethics 22, 122 (2021). https://doi.org/10.1186/s12910-021-00687-3
- Figures 1-4 designed using draw.io.
- Adapted with permission from:
- Abbasi B, Goldenholz DM. Machine learning applications in epilepsy. Epilepsia. 2019 Oct;60(10):2037-2047. doi: 10.1111/epi.16333. Epub 2019 Sep 3. PMID: 31478577; PMCID: PMC9897263.
- Zweifel, C., Lavinio, A., Steiner, L. A., Radolovich, D., Smielewski, P., Timofeev, I., Hiler, M., Balestreri, M., Kirkpatrick, P. J., Pickard, J. D., Hutchinson, P., & Czosnyka, M. (2008). Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurgical Focus FOC, 25(4), E2.
- Vamsidhar Chavakula, Iván Sánchez Fernández, Jurriaan M. Peters, Gautam Popli, William Bosl, Sanjay Rakhade, Alexander Rotenberg, Tobias Loddenkemper. Automated quantification of spikes. Epilepsy & Behavior, Volume 26, Issue 2, 2013, Pages 143-152, ISSN 1525-5050



































